- Visibility 1.1k Views
- Downloads 71 Downloads
- Permissions
- DOI 10.18231/j.ijrimcr.2024.050
-
CrossMark
- Citation
The role of medical writing in clinical research
- Author Details:
-
Abhijit Trailokya *
Abstract
Medical writing is a specialized field that involves the creation of scientific documents by writers with a detailed understanding of medical and scientific concepts. In the past few decades, medical writing has seen substantial growth in India. This field encompasses the creation of regulatory documents, safety reports, publications, and educational and communication materials pertaining to health and healthcare products. These documents are crucial in various aspects of healthcare, including clinical research, regulatory affairs, marketing, and education. Medical writing plays a crucial role in the successful execution and documentation of clinical trials. This article delves into the responsibilities of medical writers, the importance of their work in the clinical trial process, and how they ensure regulatory compliance and clear communication of clinical trial outcomes.
Introduction
Medical writing in clinical research is a specialized field that involves creating well-structured and scientifically accurate documents related to clinical research as per the requirement of regulatory authorities (DCGI, USFDA, etc.).[1] These documents are essential for the regulatory approval of new drugs, biologics, and medical devices, as well as for the dissemination of clinical trial results to the scientific community.[2], [3] Medical writers need to combine their scientific knowledge and their research understanding as well as detailed knowledge of GCP and ICH guidelines to present information at the right level for the target audience.[2] Medical writing ranks as the fourth most frequently outsourced activity in clinical development. Its global demand has consistently risen, driven by increasing cost pressures within the pharmaceutical industry.[1]
Writing for Clinical Trial Documents
Medical writers are critical to the communication process of outlining the goals, strategies, analysis, and medical understanding of a clinical trial/program to patients, sites, sponsors, and regulatory agencies.
Medical writers are involved in writing for various Clinical research documents highlighted in [Figure 1].
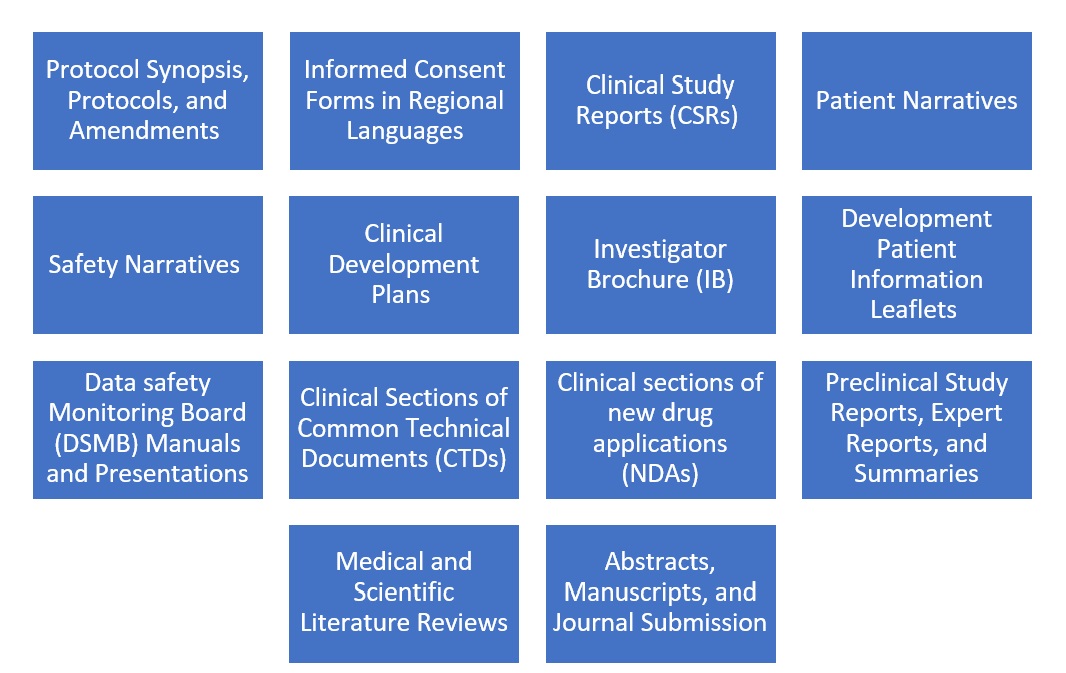
Responsibilities of Medical Writers in Clinical Trials
Responsibility starts from designing a synopsis and ends with the publication of trial results or manuscripts in a reputed journal.
Synopsis/protocol development
Medical writers were involved in developing a clinical trial synopsis followed by protocols that outline the study objectives, design, methodology, statistical considerations, and operational aspects of the clinical trial in consultation with therapy experts from the respective fields. A well-written protocol ensures that all investigators follow a standardized procedure, which is critical for the integrity of the trial data. Medical writers should have adequate knowledge and experience not only in clinical trials but also in the regulations involved in clinical research. He has to work in collaboration with therapy experts, investigators, regulatory experts, etc.
Informed consent documents
They create informed consent documents to ensure participants understand the study's purpose, procedures, risks, and benefits. Clear and comprehensive informed consent forms help protect participants' rights and ensure ethical standards are upheld.
Clinical study reports (CSRs)
A CSR is a critical document for regulatory submissions and must present data in a clear, logical, and unbiased manner. Medical writers prepare CSRs that provide a detailed account of the trial's methodology, results, and conclusions in collaboration with investigator, subject expert, statistician etc.
Regulatory submissions
Accurate and complete submissions are essential for obtaining regulatory approval to market a new drug. They compile regulatory submission documents, including Investigational New Drug (IND) applications, New Drug Applications (NDAs), and Marketing Authorization Applications (MAAs).
Manuscripts and publications
Medical writers draft manuscripts in consultation with the principal investigator, statistician, etc., for publication in peer-reviewed journals to disseminate trial findings to the broader scientific community.
Requirements for becoming medical writer for clinical research
The basic prerequisite for becoming a medical writer is familiarity with medical concepts and terminology. An academic qualification in one of the life sciences branches like medicine, or paramedical sciences such as pharmacy, science graduates and postgraduates, doctorates (PhD) in science background can provide the right background which makes the writer familiar with scientific concepts and research data. Some degree of command over the language, reflected by an ability to write grammatically correct text and an ability to express and present information clearly and succinctly, is most important. In addition to the above basic requirements, one needs domain knowledge and language skills, including: 1) Medical and therapeutic area knowledge 2) Drug development process, pharmacology, drug safety 3) Basic understanding of statistics 4) Technical guidelines in relation to clinical research and regulatory affairs 5) Interpretation and presentation of research data.
The Demand for Medical Writing in Clinical Research
The demand for medical writing has gone up considerably in the last few years. The reasons are many – more research studies are being conducted today in the biomedical field, pharmaceutical companies are developing more new drugs and medical devices, and various scientific documents need to be generated for submission to regulatory authorities during their approval process. India offers unique advantages for outsourcing medical writing services, including a large workforce of science graduates and medical professionals proficient in English, coupled with lower operational costs.[4], [5], [1]
Many leading pharmaceutical companies, including multinationals, maintain in-house medical writing teams. Several have established captive medical writing teams in India to leverage the expertise of local medical writers and address the needs of the expanding clinical trials market in the country. India has steadily attracted medical writing business from pharmaceutical companies worldwide, particularly from those in the US and UK. Most of the top 20 global pharmaceutical companies outsource their medical writing work to India.[4]
Conclusion
Medical writing is a vital component of the clinical research process, ensuring that all documentation is clear, accurate, and compliant with regulatory standards. By translating complex scientific data into understandable formats, medical writers play a key role in advancing medical research and facilitating the development of new treatments.
Source of Funding
None.
Conflict of Interest
None.
Acknowledgment
Thanks to Mr. Santosh Tompe (Senior Manager Clinical and Regulatory Affairs, Indoco Remedies Ltd., Mumbai) for helping with manuscript writing.
References
- Sharma S. Development of medical writing in India: Past, present and future. Perspect Clin Res. 2017;8(1):45-50. [Google Scholar]
- Korieth K. Demand for medical writing continues to rise. Centerwatch Mon. 2008;15(12):8-13. [Google Scholar]
- Sharma S. How to become a competent medical writer?. Perspect Clin Res. 2010;1(1):33-7. [Google Scholar]
- Shirke S. Medical writing on an accelerated path in India. Perspect Clin Res. 2015;6(3):125-8. [Google Scholar]
- Kanade M. Medical writing. . 2022. [Google Scholar]
- Abstract
- Introduction
- Writing for Clinical Trial Documents
- Responsibilities of Medical Writers in Clinical Trials
- Synopsis/protocol development
- Informed consent documents
- Clinical study reports (CSRs)
- Regulatory submissions
- Manuscripts and publications
- The Demand for Medical Writing in Clinical Research
- Conclusion
- Source of Funding
- Conflict of Interest
- Acknowledgment
- References
How to Cite This Article
Vancouver
Trailokya A. The role of medical writing in clinical research [Internet]. Int J Recent Innov Med Clin Res. 2024 [cited 2025 Nov 06];6(2):53-55. Available from: https://doi.org/10.18231/j.ijrimcr.2024.050
APA
Trailokya, A. (2024). The role of medical writing in clinical research. Int J Recent Innov Med Clin Res, 6(2), 53-55. https://doi.org/10.18231/j.ijrimcr.2024.050
MLA
Trailokya, Abhijit. "The role of medical writing in clinical research." Int J Recent Innov Med Clin Res, vol. 6, no. 2, 2024, pp. 53-55. https://doi.org/10.18231/j.ijrimcr.2024.050
Chicago
Trailokya, A.. "The role of medical writing in clinical research." Int J Recent Innov Med Clin Res 6, no. 2 (2024): 53-55. https://doi.org/10.18231/j.ijrimcr.2024.050
