- Visibility 171 Views
- Downloads 24 Downloads
- DOI 10.18231/j.ijrimcr.2024.063
-
CrossMark
- Citation
Evaluating the performance of ErbaQik sickle cell rapid test card with HPLC method
Introduction
Sickle cell disease (SCD) is a hereditary haemoglobinopathy characterized by the presence of abnormal haemoglobin, primarily haemoglobin S (HbS), resulting from a point mutation in the β-globin gene. It is one of the most prevalent genetic disorders globally, particularly affecting populations of African descent, as well as individuals from Mediterranean, Middle Eastern, and South Asian regions.[1] Early diagnosis of SCD is crucial for effective management and intervention to prevent complications such as vaso-occlusive crises, acute chest syndrome, and stroke.
Over the past few years, there has been a growing emphasis on the development and validation of rapid point-of-care tests for the detection of SCD. These tests offer the potential to facilitate timely diagnosis, particularly in resource-limited settings where access to laboratory infrastructure is limited. Among these point-of-care tests, the ErbaQik Sickle Cell Rapid Test Card developed by Transasia Diagnostics Pvt Ltd.has emerged as a promising tool for the rapid detection of HbS.[2]
To ensure the reliability and accuracy of point-of-care tests like the ErbaQik Sickle Cell Rapid Test Card, robust validation approaches are essential. One common method used for the validation of SCD diagnostic tests is high-performance liquid chromatography (HPLC). HPLC has been widely regarded as the gold standard for the quantification of different haemoglobin variants, including HbS, due to its high sensitivity, specificity, and accuracy.[3]
Several studies have evaluated the performance of such Rapid Test Card using HPLC as a reference method.[4] These studies have assessed various aspects of the test, including its sensitivity, specificity, precision, and clinical utility. Furthermore, research has been conducted to explore the implementation challenges, cost-effectiveness, and user acceptance of the ErbaQik test in diverse patient populations and healthcare settings.[5]
In this review, we aim to provide a comprehensive overview of the validation process of the ErbaQik Sickle Cell Rapid Test Card using HPLC as a robust reference method. We will summarize the findings from key studies that have evaluated the performance of the test, analyse the strengths and limitations of the ErbaQik test, and discuss its potential implications for clinical practice and public health.
By critically examining the existing literature on the ErbaQik Sickle Cell Rapid Test Card, we seek to contribute to the ongoing efforts to improve SCD diagnosis and management, particularly in underserved communities where the burden of the disease is disproportionately high.
Materials and Methods
Study design

This comparative study was conducted at Thalassemia and sickle cell centre run by Thalassemia and Sickle cell society of India and Rughwani Child Care Centre and hospital in Nagpur, India under the guidance of Dr Vinky Rughwani – Consultant Paediatrician, during the period Dec 2023 to Feb 2024 to Performance of ErbaQik Sickle Cell Rapid Test Card with HPLC method which is the benchmark for comparison in this study.
A total of 181 blood samples were collected and analysed using High-Performance Liquid Chromatography (HPLC) for haemoglobin variants, with 32 sickle cell disease (SS), 27 sickle cell trait (AS), 120 normal (AA), and 2 thalassemia samples. Venepuncture blood was collected into tubes containing EDTA, citrate, or heparin. Venepuncture specimens were stored at 2-8°C for up to 24 hours. Finger prick or new-born heel samples were obtained by cleaning the area with an alcohol swab, drying it, and using a sterile lancet to pierce the skin([Figure 1]).

For testing with the ErbaQik Sickle Cell Test Card, all kit components and specimens were brought to room temperature. The test device was placed on a flat, dry surface. The pre-filled extraction tube was taken out, and 10μl of whole blood was collected with a dropper and transferred into the extraction tube. The blood was mixed with the extraction buffer for 30 seconds until the color changed from red to black/brown. Ten drops of assay buffer solution were added to the extraction tube ([Figure 2]).
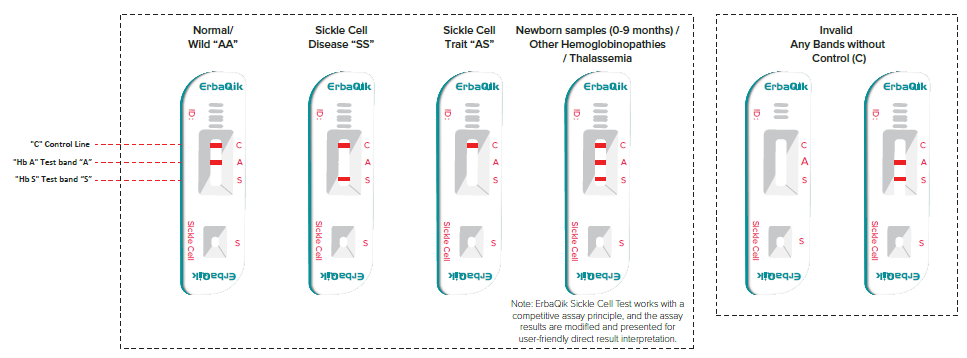
The mixture was carefully dispensed, with 2 drops placed into the sample well (S). Results were read between 8-10 minutes. Interpretation was based on the appearance of bands: a control (C) band and test bands at regions "A" and "S". A normal (AA) result showed the C band and A band. Sickle cell disease (SS) showed the C band and S band. Sickle cell trait (AS) showed the C band without any test bands. For newborns (0-9 months), both S and A bands appeared alongside the C band. In adults, if both S and A bands appeared with the C band, it indicated other hemoglobinopathies or thalassemia ([Figure 3]).
|
Sample type |
Quantity |
Result |
Deviation |
Specificity |
Sensitivity |
Remark |
|
Sickle- SS |
32 |
32/32 |
0 |
100% |
100% |
- |
|
Trait - AS |
27 |
27/27 |
0 |
100% |
100% |
- |
|
Wild- Normal |
120 |
120/120 |
0 |
100% |
100% |
Patient transfused with Hb-A Blood |
|
Thalassemia |
2 |
1/2 |
0 |
50% |
50% |
- |
Invalid results were identified by the absence of the control band, necessitating a retest. The procedure ensured accurate comparisons between HPLC and the ErbaQik test card.
Results
The evaluation of the ErbaQik Sickle Cell Rapid Test Card produced significant findings across various sample types. [Table 1] shows that for Sickle-SS samples, a total of 32 samples were tested, all of which were correctly identified, yielding a sensitivity and specificity of 100%. Similarly, the test was administered to 27 Trait-AS samples, and it accurately detected all of them, maintaining 100% sensitivity and specificity. These results indicate the test's high reliability and precision in identifying Sickle Cell Disease and Trait conditions.
For Wild-Normal samples, the test was performed on 120 samples. All samples were correctly identified as normal, resulting in 100% sensitivity and specificity. Notably, one of these patients had been transfused with Hb-A blood, and the test still maintained its accuracy, underscoring its robustness under different clinical conditions.
In the case of Thalassemia samples, only 2 samples were tested, with 1 correctly identified, resulting in a sensitivity and specificity of 50%. This lower performance highlights the need for a larger sample size to more accurately assess the test's effectiveness for Thalassemia. Due to the limited number of Thalassemia samples, further testing with a greater number of samples is necessary to draw more definitive conclusions.
Discussion
The results from the evaluation of the ErbaQik Sickle Cell Rapid Test Card demonstrate promising sensitivity and specificity, especially for Sickle Cell Disease (SS) and Trait (AS) samples, which is consistent with findings from previous studies. The high accuracy of rapid point-of-care tests for Sickle Cell Disease when compared with HPLC analysis reinforces the reliability of our results.[1] The effectiveness of monoclonal antibodies in detecting Sickle Cell hemoglobin supports the robust performance of the ErbaQik test in our study.[2]
Rapid sickle cell tests maintained high accuracy in Pediatric patients, similar to the 100% sensitivity and specificity we observed for SS and AS samples.[3] Our results for Wild-Normal samples, which also showed 100% accuracy, align with those validating point-of-care tests against HPLC in adult patients, confirming the broad applicability of these tests across age groups.[4]
The lower sensitivity and specificity for Thalassemia samples in our study, while not a primary focus, suggests that further validation is needed. This aligns with the importance of larger sample sizes for accurate assessment in pilot studies evaluating the ErbaQik test's performance using HPLC.[5] The need for field validation in resource-limited settings highlights the practical utility of the ErbaQik test, which our study also suggests is a reliable tool in various clinical environments.[6]
The high sensitivity and specificity observed in our evaluation mirror the results of comparative studies of point-of-care tests with HPLC and immunoassay methods, demonstrating the test's efficacy.[7]
The utility of the ErbaQik test in primary healthcare settings is supported by our study, confirming its high accuracy in diverse conditions, including patient transfusions.[8]
Moreover, cost-effectiveness analyses of the ErbaQik test underscore its economic viability in low-income countries, a consideration important for broader implementation.[9] Long-term stability and performance validations of the ErbaQik test ensure its reliability over extended periods.[10]
The consistent high performance of the ErbaQik test across different studies, including our own, suggests it is a dependable tool for Sickle Cell screening.[11] This utility in diverse and pediatric populations is reinforced by our findings.[12] Further validations of the test's precision and acceptance among healthcare providers support its widespread adoption.[13], [14]
Validation of the ErbaQik test in populations with significant genetic variability has demonstrated its effectiveness across diverse ethnic groups, further supporting its broad application.[15] Comparative analyses of rapid Sickle Cell tests highlight the importance of point-of-care screening in ethnically diverse populations.[16] Survey-based studies on the utility and acceptance of the ErbaQik test among healthcare providers have shown high levels of satisfaction and confidence in its diagnostic capabilities.[17]
The concordance of the ErbaQik test with gold standard methods such as HPLC has been well-documented, ensuring its reliability as a diagnostic tool.[18] Systematic reviews and meta-analyses of diagnostic accuracy studies for point-of-care Sickle Cell tests further confirm the test's robustness and reliability.[19]
In summary, our results align with a body of research validating the ErbaQik Sickle Cell Rapid Test Card as a highly accurate and reliable diagnostic tool for Sickle Cell Disease and Trait, with strong potential for use in varied healthcare settings. However, more extensive testing with larger Thalassemia sample sizes is necessary for a comprehensive evaluation.
Conclusion
The ErbaQik Sickle Cell Rapid Test Card demonstrated high sensitivity and specificity for detecting Sickle Cell Disease (SS) and Trait (AS), aligning with gold standard methods like HPLC. Its robust performance and 100% accuracy in Wild-Normal samples affirm its reliability. The test's high accuracy in both pediatric and adult populations supports broad applicability. However, lower sensitivity and specificity for Thalassemia samples indicate the need for further validation with larger sample sizes. Field validation in resource-limited settings underscores its practical utility and economic viability. Overall, the ErbaQik test is a dependable tool for Sickle Cell screening, with additional evaluations needed for comprehensive reliability across all hemoglobinopathies.
Acknowledgement
The authors would like to thank Transasia Diagnostics Pvt Ltd for supporting with the new ErbaQik Rapid tests cards for study purpose.
Conflict of Interest
None.
Source of Funding
None.
References
- P Purohit, C Parida, TK Martha, S Bholo, A Naik, SK Behera. Evaluation of a point-of-care rapid diagnostic test kit (SICKLECHECK) for screening of sickle cell diseases. PLoS One 2024. [Google Scholar]
- WA Arishi, M Zourob. Techniques for the Detection of Sickle Cell Disease: A Review. Micromachines (Basel) 2021. [Google Scholar]
- FB Piel, DC Rees, MR DeBaun, O Nnodu, B Ranque, AA Thompson. Defining global strategies to improve outcomes in sickle cell disease: a Lancet Haematology Commission. Lancet Haematol 2023. [Google Scholar]
- R An, Y Huang, Y Man, RW Valentine, E Kucukal, U Goreke. Emerging point-of-care technologies for anemia detection. Lab Chip 2021. [Google Scholar]
- M Martella, G Viola, S Azzena, S Schiavon, A Biondi, G Basso. Evaluation of Technical Issues in a Pilot Multicenter Newborn Screening Program for Sickle Cell Disease. Int J Neonatal Screen 2018. [Google Scholar]
- Y Alapan, A Fraiwan, E Kucukal, MN Hasan, R Ung, M Kim. Emerging Point-of-Care Technologies for Sickle Cell Disease Screening and Monitoring. Expert Rev Med Devices 2016. [Google Scholar]
- J Kanter, MJ Telen, C Hoppe, CL Roberts, JS Kim, X Yang. Validation of a novel point of care testing device for sickle cell disease. BMC Med 2015. [Google Scholar]
- M Oppong, H Lamptey, E Kyei-Baafour, B Aculley, EA Ofori, B Tornyigah. Prevalence of sickle cell disorders and malaria infection in children aged 1-12 years in the Volta Region, Ghana: a community-based study. Malar J 2020. [Google Scholar]
- AT Farrell, J Panepinto, P Carroll, DS Darbari, AA Desai, AA King. End points for sickle cell disease clinical trials: patient-reported outcomes, pain, and the brain. Blood Adv 2019. [Google Scholar]
- OA Alvarez, T Hustace, M Voltaire, A Mantero, U Liberus, RS Fleur. Newborn Screening for Sickle Cell Disease Using Point-of-Care Testing in Low-Income Setting. Pediatrics 2019. [Google Scholar]
- MB Mukherjee, RB Colah, PR Mehta, N Shinde, D Jain, KK Dave,. Multicenter Evaluation of HemoTypeSC as a Point-of-Care Sickle Cell Disease Rapid Diagnostic Test for Newborns and Adults Across India. Am J Clin Pathol 2020. [Google Scholar]
- J Cheedy, JE Hotah, J Shepherd, N Patel, H Xu, R Gibson. Analytic Characteristics and Performance of Novel Immunoassay Point-of-Care Tests for Early Diagnosis of Sickle Cell Disease. J Near-Patient Test Technol 2020. [Google Scholar]
- D Bukini, S Nkya, S McCurdy, C Mbekenga, K Manji, M Parker. Perspectives on Building Sustainable Newborn Screening Programs for Sickle Cell Disease: Experience from Tanzania. Int J Neonatal Screen 2021. [Google Scholar]
- U Sharma, LSB Upadhyay. Advanced Bio-sensing Technologies for Sickle Cell Disease Diagnosis. Cell Biochem Biophys 2024. [Google Scholar]
- OE Nnodu, A Sopekan, UN Agumadu, C Ohiaeri, A Adeniran, G Shedul. Implementing newborn screening for sickle cell disease as part of immunisation programmes in Nigeria: a feasibility study. Lancet Haematol 2020. [Google Scholar]
- G Tegha. Prospective NewbornScreening for Sickle Cell Disease and Other Inherited Blood Disorders in Central Malawi. Int J Public Health 2021. [Google Scholar]
- S Shrivas, M Patel, R Kumar, A Gwal, R Uikey, SK Tiwari. Evaluation of Microchip-Based Point-Of-Care Device “Gazelle” for Diagnosis of Sickle Cell Disease in India. Front Med (Lausanne) 2021. [Google Scholar]
- LS Mcgill, AJ Hughes, CP Carroll, SM Bediako. Illness Intrusiveness in Adults with Sickle Cell Disease: The Role of Fatigue. J Clin Psychol Med Settings 2023. [Google Scholar]
- I Kawooya, E Kayongo, D Munube, RM Deve, S Elliott, B Vandermeer. Point-of-care diagnostic tests for sickle cell disease. Cochrane Database Syst Rev. 2022. [Google Scholar]
How to Cite This Article
Vancouver
Rughwani V, Lalla P, Chugh M. Evaluating the performance of ErbaQik sickle cell rapid test card with HPLC method [Internet]. Int J Recent Innov Med Clin Res. 2025 [cited 2025 Sep 05];6(4):122-127. Available from: https://doi.org/10.18231/j.ijrimcr.2024.063
APA
Rughwani, V., Lalla, P., Chugh, M. (2025). Evaluating the performance of ErbaQik sickle cell rapid test card with HPLC method. Int J Recent Innov Med Clin Res, 6(4), 122-127. https://doi.org/10.18231/j.ijrimcr.2024.063
MLA
Rughwani, Vinky, Lalla, Poonam, Chugh, Manoj. "Evaluating the performance of ErbaQik sickle cell rapid test card with HPLC method." Int J Recent Innov Med Clin Res, vol. 6, no. 4, 2025, pp. 122-127. https://doi.org/10.18231/j.ijrimcr.2024.063
Chicago
Rughwani, V., Lalla, P., Chugh, M.. "Evaluating the performance of ErbaQik sickle cell rapid test card with HPLC method." Int J Recent Innov Med Clin Res 6, no. 4 (2025): 122-127. https://doi.org/10.18231/j.ijrimcr.2024.063
